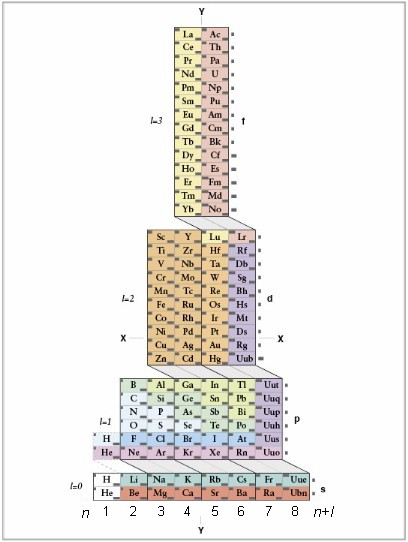
Most people with some background in science are familiar with the Periodic Table. It can be found in many textbooks that deal with chemistry and physics. Although it's traditional depiction presents the periodicity of chemical properties of the elements very well, it does not, however, properly reflect the Electronic Structure of Atoms, and so lacks the consistency expected from something often regarded as "The Symbol of Science".
Dmitri Mendeleev in his article "The Relations Between the Properties of Elements and Their Atomic Weights" wrote following: "...every system, however, that is based upon exactly observed numbers is to be preferred, of course, to other systems not based upon numbers because then only little margin is left to arbitrariness... Properties, such as the optical and even the electrical or magnetic ones, cannot serve as basis for the system naturally, since one and the same body, according to the state in which it happens to be at the moment, may show enormous differences in this regard." This is exactly what is wrong with the traditional periodic table that "cuts" the sequence of the elements in periods primarily on the basis of metallic/nonmetallic/inert properties.
Hydrogen is a good example why Periodic Table should not be based on such principles. It makes up more than 90% of all the atoms in the Universe and it can be regarded either as metal, or as nonmetal. Another example is element 114 (Uuq) that was expected to resemble lead (Pb), but, according to recent reports, behaves as a noble gas.
But what could be the "exactly observed numbers" that Mendeleev alluded to? One of such numbers would obviously be the Atomic Number Z. However, if only this number is used, the Periodic Table would take a form of a continuous line or a column. Other "exactly observed numbers" are necessary for the correct representation of the Periodic Law. The best choice, of course, would be the Quantum Numbers, which were discovered through the means of spectroscopy.
Mendeleev did not know about the quantum numbers, therefore, he had to use what was available to him at the time of his inquiry: atomic weights and the properties of the elements. The quotation presented above makes it absolutely clear that, had he known about the quantum numbers, he would most likely use them for the Periodic Table formulation.
Two of the four quantum numbers, "n" and "l", are reflected in the standard periodic table. However, only first three periods of the traditional periodic table follow the quantum number "n". Beginning with the forth period the pattern breaks down and the periods no longer correspond to the quantum number "n". Major areas of the traditional periodic table, that are also called s,f,d,p blocks, are presented in order of l = 0, 3, 2, 1 instead of l = 0, 1, 2, 3. Consequently, in accordance with the Mendeleev's statement presented above, the architecture of the traditional Periodic Table is arbitrary and does not represent the natural limits of the periods. The natural limits can be determined using following procedure:
Step 1. For each chemical element determine the maximum "n+l" value attained by its electron(s) in ground state;
Step 2. List all (n+l)max values obtained in Step 1 in order of the atomic number Z. Below is (n+l)max sequence for the first 120 elements:
112233333333444444445555555555555555556666666666666666667777777777777777777777777777777788888888888888888888888888888888.
Looking at (n+l)max list above, one can observe that the sequence of the chemical elements listed in order of Z has naturally defined groups:
11, 22, 33333333, 44444444, 555555555555555555, 666666666666666666, 77777777777777777777777777777777, 88888888888888888888888888888888
Therefore, by counting number of integers in each group shown above, we can determine the correct lengths of the periods, which are as follows:
2, 2, 8, 8, 18, 18, 32, 32 as was shown by Janet in 1920's, when he introduced his Left Step Periodic Table.
The standard IUPAC PT formulation has period lengths of 2, 8, 8, 18, 18, 32, 32. Its first line, that comprises two elements H and He, does not recur and, therefore, is not a period. Consequently, the IUPAC standard Periodic Table is not truly periodic. Below is how elements are grouped in the standard periodic table in relation to (n+l)max:
11, 22333333, 33444444, 445555555555555555, 556666666666666666, 66777777777777777777777777777777, 77888888888888888888888888888888,
this is a violation of the natural grouping.
The above approach used for determination of the natural lengths of the periods also answers few other questions, such as placement of H, He, La and Ac, objectively. One might ask why "n+l" values are used? The answer is: 1)quantum numbers "n" and "l" define electronic shells and subshells and, 2)empirically derived Madelung rule, also known as "n+l" rule, reflects general ordering of electron orbital energies within atoms.
Another deficiency of the standard table is that the group of 28 elements is separated from the main body of the table and turned into a footnote for the sole purpose of reducing PT's disproportional length, that could have also been addressed from the quantum perspective.
Perhaps this is the right time to consider seriously the correct representation of the Periodic System, the one that closely follows the quantum numbers and the atomic structure. ADOMAH Periodic Table is such table and you can learn more about it here.
There are hundreds of other tables besides the standard "periodic" table. Some of them "cut" the sequence of the elements at even more peculiar places than the standard table does. Only Janet's LSPT and ADOMAH PT presented here follow natural grouping of the elements and only ADOMAH PT allows direct readout of the quantum number "n" in addition to "l" and "n+l".
The urge to change the current standard is understandable. It is far from perfect and is awkward to use when it comes to determining electron configurations, which are, by all means, more important for understanding of nature of the elements, than grouping them by their metallic and non metallic properties. On the other hand, it is not that simple to arrange the elements in accordance with electron configurations. Therefore, it is important to set forth parameters for such formulation.
The periodic nature of chemical elements is direct outcome of their complex atomic structure. Therefore, in order to arrange them correctly, five main requirements must be met:
Copyright 2008 by Valery Tsimmerman. All rights reserved.
Major updates: 04/06/08, 04/27/08, 12/04/08, 03/19/09